Hallo sobat100, belajar bersama Seratusinstitute.com....
Yuk simak soal berikut :
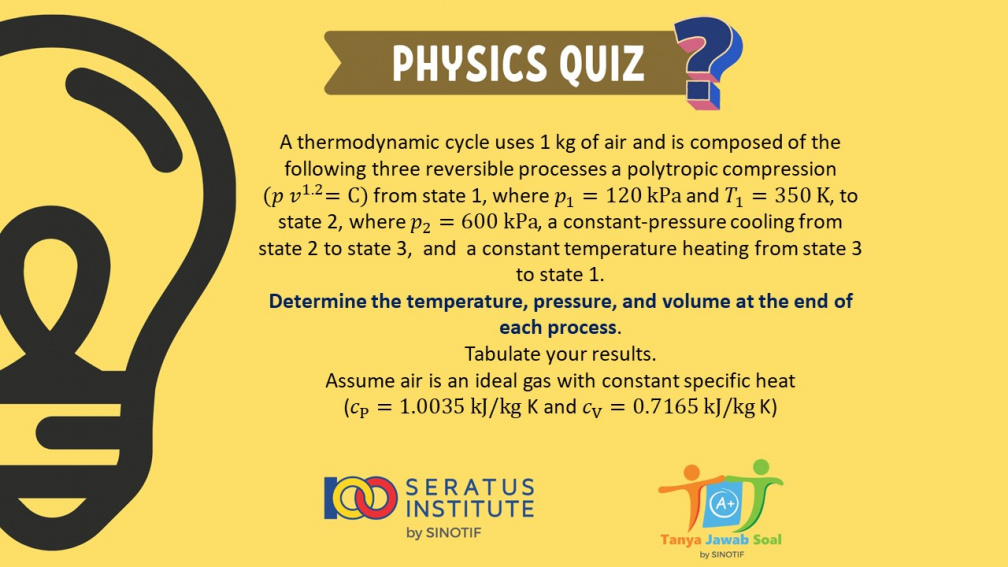
A thermodynamic cycle uses 1 kg of air and is composed of the following three reversible processes a polytropic compression ( 𝑝 𝑣1.2 = C ) from state 1, where 𝑝1 = 120 kPa and 𝑇1 = 350 K, to state 2, where 𝑝2 = 600 kPa, a constant-pressure cooling from state 2 to state 3, and a constant temperature heating from state 3 to state 1.
Determine the temperature, pressure, and volume at the end of each process.
Tabulate your results. Assume air is an ideal gas with constant specific heat (𝑐_P=1.0035 kJ/kg K and 𝑐_V=0.7165 kJ/kg K)
Berikut pembahasannya..
Jika sobat100, punya tugas/PR yang ingin kamu tanyakan kamu bisa gunakan aplikasi TANYA JAWAB SOAL
Download Aplikasinya Sekarang di Playstore!
Atau link di bawah ini:
Belajar Jadi Mudah dan Menyenangkan





Komentar berhasil disembunyikan.